CUE-101
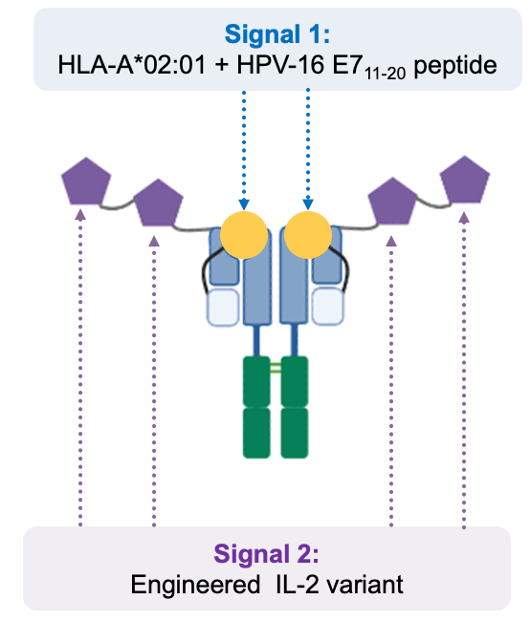
CUE-101 is Cue Biopharma’s lead drug product candidate currently being evaluated for the treatment of human papilloma virus (HPV16+)-driven recurrent/metastatic head and neck cancer.
Signal #1 incorporates the HPV E7 protein, harbored by HPV-induced cancer cells, and Signal #2 consists of the engineered IL-2 variant. As such, it is designed to activate and expand HPV16 tumor-specific T cells.
CUE-101 in the Clinic
CUE-101 as a Monotherapy
CUE 101 is currently being evaluated in a Phase 1 trial for the treatment of second line and beyond treatment for human papilloma virus positive recurrent/metastatic head and neck squamous cell carcinoma (HPV+ R/M HNSCC).
In this highly pretreated patient population, CUE-101 has demonstrated significant clinical benefit to date, a rarity for the immuno-oncology space in which very few immunotherapies show single-agent clinical benefit.
The observed clinical activity of CUE-101 as a monotherapy establishes initial proof of concept for the potential of Cue Biopharma’s CUE-100 series of IL-2 based biologics for the treatment of cancer.
Learn more about the CUE-101 monotherapy trial.
CUE-101 + Pembrolizumab
The company is additionally advancing CUE-101 through a Phase 1 dose escalation and expansion trial in combination with the anti-PD-1 checkpoint inhibitor pembrolizumab (KEYTRUDA®), as first-line treatment in the same patient population.
Learn more about the CUE-101 combination trial.
CUE-101 in the Neoadjuvant Setting
CUE-101 is additionally being evaluated in a Phase 2 investigator-sponsored trial administered as a neoadjuvant (prior to surgery or chemo-radiation therapy), in treatment naïve, HLA-A*0201 positive patients with newly diagnosed, locally advanced, HPV16+ oropharyngeal squamous-cell carcinoma (OPSCC).
Learn more about the CUE-101 neoadjuvant trial.
CUE-102
CUE-102, Cue Biopharma’s second drug product candidate, is designed to activate and expand tumor-specific T cells that target Wilms’ Tumor 1 (WT1)-expressing cancers. Signal #1 incorporates a WT1 peptide, and Signal #2 consists of the engineered IL-2 variant.
CUE-102 in the Clinic
CUE -102 is being evaluated in a dose escalation and expansion monotherapy Phase 1 trial in the U.S., at a starting dose of 1mg/kg, with initial focus on gastric, pancreatic, ovarian and colon cancers.
Learn more about the CUE-102 monotherapy trial.
CUE-103
CUE-103 will leverage the CUE-100 framework.
Versatile Pipeline
Immuno-STAT biologics can be further engineered and developed for the treatment of indications other than cancer, including infectious and autoimmune diseases, by swapping out Signal #1 for self-specific or pathogen-specific antigens and Signal #2 for immune-inhibitory signals (in the case of autoimmune disease).
To learn more, visit our Partnering section.

