The Immuno-STAT™ (Selective Targeting and Alteration of T cells) Platform
Cue Biopharma’s Immuno-STAT platform enables engineering of stable, off-the-shelf molecules that mimic the natural mechanism that antigen presenting cells (APCs) – specialized immune cells in charge of presentation of foreign proteins to the immune system – use to engage tumor-specific “killer” T cells during an immune response, through the presentation of two simultaneous signals or “cues.”
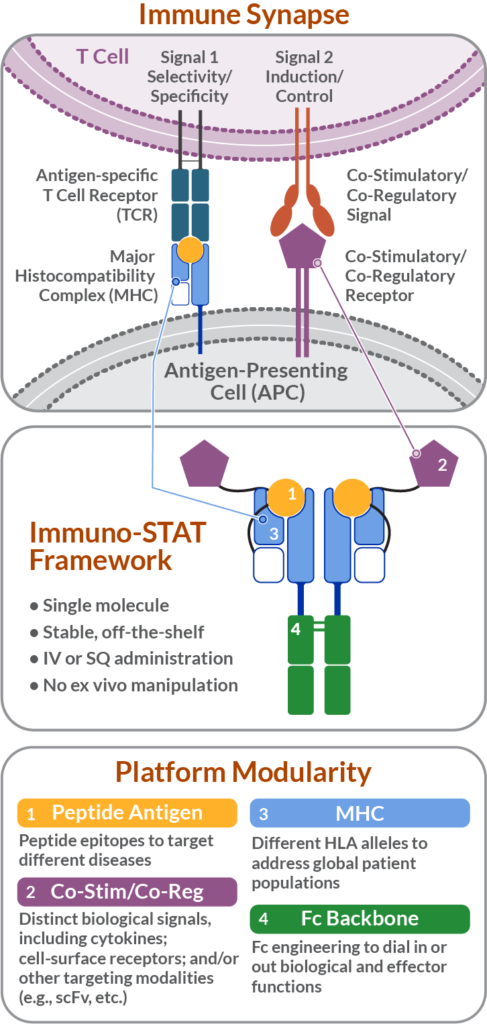
Immuno-STAT Framework
Signal #1: Enables selectivity and specificity
Signal #1 involves the presentation of tumor-specific proteins, via a major histocompatibility peptide complex (pMHC), to T cell receptors of the target tumor-specific T cells, to modulate the repertoire of T cells for the anti-cancer response.
Signal#2: Modulates activation or inhibition of immune response
Signal #2 consists of a key immune-stimulatory signal, interleukin 2 (IL-2) to enable selective activation of tumor-specific “cancer killing” T cells for the treatment of cancer.
MHC: The major histocompatibility complex, a specific ID sequence for a cell or tissue, can be engineered with different disease-specific human leukocyte antigen (HLA) alleles to address diverse patient populations.
Fc Backbone: Immuno-STATs are constructed upon a portion of a human antibody that serves as the molecule’s backbone or scaffold (the “Fc portion”) and provides manufacturability and structural stability that can be engineered to dial in or out biological and effector functions.
Key Benefits of the Immuno-STAT Framework
- Tumor specificity: Due to the unique engineered MHC component for the presentation of tumor specific peptides, Cue Biopharma’s Immuno-STAT biologics selectively activate tumor-specific T cells.
- Selective activation of CD8+ “cancer killer” T cells: Tumor-specific Immuno-STATs include a carefully engineered IL-2 molecule, which is biased toward activation of CD8+ cytotoxic (“killer”) T effector cells and avoid broad activation of T regulatory cells (Tregs). Learn more about Cue Biopharma’s engineered IL-2 molecule.
- A larger therapeutic window for IL-2 effectiveness: Immuno-STAT IL-2 based biologics have greatly reduced the toxicities seen with other IL-2-based therapies. In clinical testing to date, CUE-101 has yet to show dose-limiting toxicities in dose escalation while already showing rare monotherapy clinical activity.
- Immune modulation: Immuno-STATs do not block a specific enzymatic reaction, biochemical pathway or receptor-ligand interaction as do many chemotherapeutic and targeted agents as well as most antibody-based therapeutics. Rather, Immuno-STATs stimulate and activate a patient’s adaptive immune response.
- No need for ex-vivo manipulation: Immuno-STATs present advantages over other tumor-specific immuno-therapies in development such as CAR-T (chimeric antigen receptor T cells) or TCR-T (T cell receptor-engineered T cell) therapies, since Immuno-STATs do not require ex vivo manipulation.
- Disease versatility: Immuno-STAT biologics can be further engineered for the treatment of indications other than cancer, including autoimmune and infectious diseases, by swapping out Signal #1 for self-specific or pathogen-specific antigens and Signal #2 for immune-inhibitory signals (in the case of autoimmune disease). To learn more, visit our Partnering section.
Learn more about Immuno-STAT in articles published in the Nature Journal, Scientific Reports and in Drug Delivery and Development.

