Attractive Opportunities in Infectious and Autoimmune Diseases
Immuno-STAT biologics can be further engineered and developed for the treatment of indications other than cancer, including autoimmune and infectious diseases, by swapping out Signal #1 for self-specific or pathogen-specific antigens and Signal #2 for immune-inhibitory signals (in the case of autoimmune disease).
Cue Biopharma’s Infectious Disease Opportunity
CUE-200 Series
The CUE-200 series biologics are engineered to boost the natural immune response to control and potentially clear chronic viral infections. CUE-200 biologics selectively deliver the required primary and co-stimulatory signals needed to further activate and expand virus-specific CD8+ cytotoxic or “killer” T cells within the patient’s body. Therapeutic approaches to date have only achieved this with ex vivo manipulation of immune cells, which limits their clinical applicability.
Preclinical research using mice with a humanized immune system demonstrated the ability of Cue Biopharma’s molecules to selectively reactivate and expand human immunodeficiency virus (HIV) and cytomegalovirus (CMV)-specific CD8+ cytotoxic T cells and suppressed viral infection. These findings support the potential use of CUE-200 biologics for the treatment of a diversity of chronic infectious diseases, including potentially achieving a functional cure for HIV infection.
Cue Biopharma welcomes your interest in partnering opportunities for our infectious disease programs.
For inquiries, please contact us at BD@cuebio.com.
Current Academic Collaborations in Infectious Disease

Cue Biopharma entered into agreements with the Albert Einstein College of Medicine (Einstein) under which Einstein will research Neo-STAT biologics for treating conditions relating to pathogenic infections, including chronic infectious diseases. Einstein’s research will leverage the versatility of the Neo-STAT platform to expand the therapeutic reach of Neo-STATs beyond cancer to infectious diseases.
Cue Biopharma’s Autoimmune Disease Opportunity
Cue Biopharma’s opportunity in autoimmunity and inflammation is centered on three key approaches:
Antigen-specific approach (CUE-300 Series): Focuses on diseases with well characterized autoantigens – Cue Biopharma’s Immuno-STAT biologics are engineered to directly modulate the antigen-specific overreactive T cells that attack self-tissue.
Pathway-specific approach (CUE-400 Series): Focuses on diseases that lack known antigens – It leverages Cue Biopharma’s biologics that modulate regulatory T cells and pathways that restore immune balance to avoid attack of self-tissue. Tregs are a critical subset of immune cells involved in the control of immune tolerance by regulating immune homeostasis and limiting self-reactive immune responses.
B cell-specific (CUE-500 Series): Focuses on autoimmune diseases caused by autoreactive, or self-reactive B cells – Cue Biopharma’s 500 series Immuno-STAT biologics are engineered to enable the patient’s anti-viral T cells to attack and destroy B cells enabling restoration of immune balance.
CUE-300 Series
The CUE-300 series of biologics are engineered to 1) selectively inhibit the activity of autoreactive CD4+ immune T cells by delivering inhibitory signals, and / or 2) enhance the activation and expansion of antigen-specific CD4+ regulatory T cells (Tregs) to control aberrant activation of autoreactive T cells.
>> Read More
CUE 301 – Type 1 diabetes
CUE-301 is designed to target and selectively inhibit proinsulin reactive CD4+ T cells in type 1 diabetes to prevent these cells from injuring or killing insulin-producing beta cells in the pancreas. Proinsulin is a prohormone precursor to insulin synthesized by beta cells of the islets of Langerhans of the pancreas, which has been recognized as an important target antigen in type 1 diabetes.
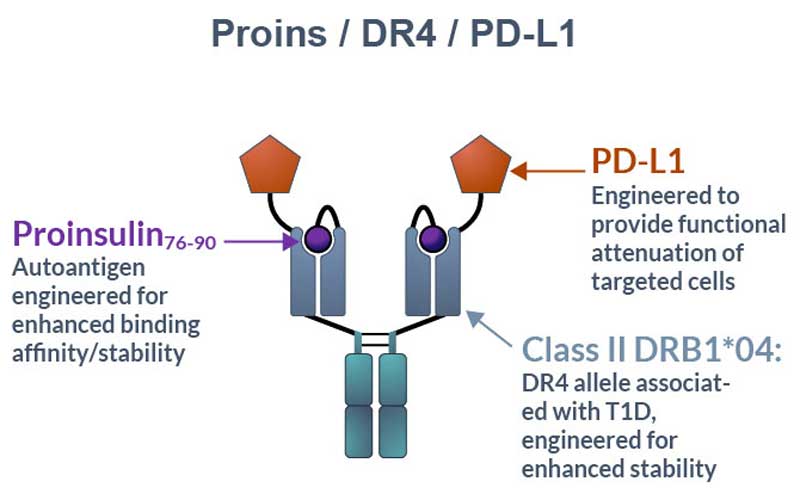
CUE-301 Presents Two Signals to T cells
Signal#1: Involves the presentation of a peptide derived from proinsulin (amino acids 76-90)
Signal#2: Includes two copies of the programmed death receptor ligand-1 (PD-L1), an immune inhibitory signal, designed to provide functional attenuation of disease-relevant T cells
CUE-301 was developed as part of a collaboration with Merck & Co., Inc., where in preclinical studies to date, CUE-301 has demonstrated to inhibit the functional in vivo expansion of proinsulin-specific CD4+ T cells along with a notable reduction in production of pro-inflammatory cytokines.
Access more information on CUE-301.
>> Close
CUE-400 Series
Cue Biopharma’s lead CUE-400 series candidate, CUE-401, is designed to selectively differentiate and expand induced CD4+FOXP3+ regulatory immune T cells (iTregs) in a patient’s body. FOXP3 iTregs are critical in maintaining immune tolerance and homeostasis of the immune system.
>>Read More
CUE-401 is a novel bispecific molecule engineered to deliver two key immunomodulatory cytokine signals known to induce differentiation of naïve immune T cells to iTregs:
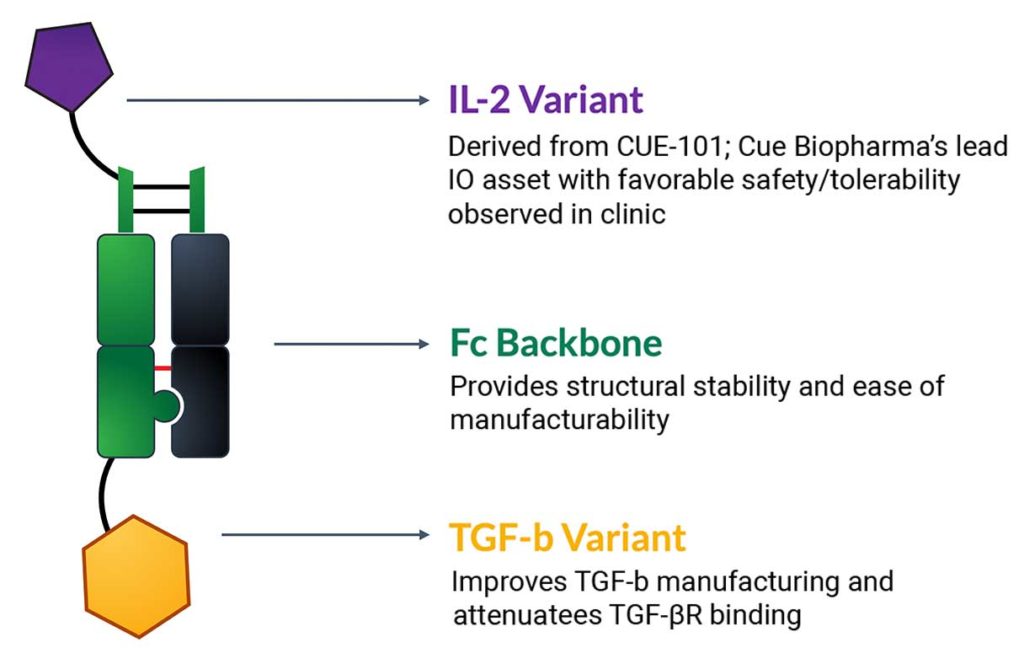
Signal #1: Transforming growth factor-beta (TGF-β), modified to reduce TGF-β receptor binding
Signal #2: Engineered interleukin 2 (IL-2) modified to have different affinity for IL-2 receptor subunits
Challenges of delivery of wild-type TGF-β and IL-2:
- It does not ensure that both signals will engage the same target T cells
- Ex vivo delivery can lead to systemic effects and safety liabilities.
CUE-401’s opportunity:
- CUE-401 provides an elegant solution by delivering the two key cytokine signals to the same target T cell – preclinical research has demonstrated that CUE-401-induced Tregs can functionally suppress effector T cell responses, supporting mechanistic activity.
- CUE-401 can potentially circumvent safety liabilities.
- CUE-401 has the potential to be deployed for many chronic autoimmune diseases like lupus, inflammatory bowel diseases, rheumatoid arthritis, and for patients suffering graft versus host disease or transplant rejection.
Cue Biopharma continues its ongoing characterization of CUE-401 in preclinical disease models to identify priority indications.
Read more about this research.
>> Close
CUE-500 Series
Cue Biopharma’s lead CUE-500 series candidate, CUE-501, is a bispecific designed to direct selective memory T cells to deplete B cells to address autoimmune and inflammatory diseases.
>> Read More
CUE-501 is designed to selectively harness the protective anti-viral T cell repertoire (virus-specific T cells, or VSTs) and redirect them to target and deplete B cells. Deploying a biologic to selectively redirect “killer” T cells, while avoiding the systemic engagement and activation of all T cells, to accomplish T cell-mediated B cell depletion addresses the important mechanism of autoimmune diseases and may offer significant advantages over current cell therapy-based strategies.
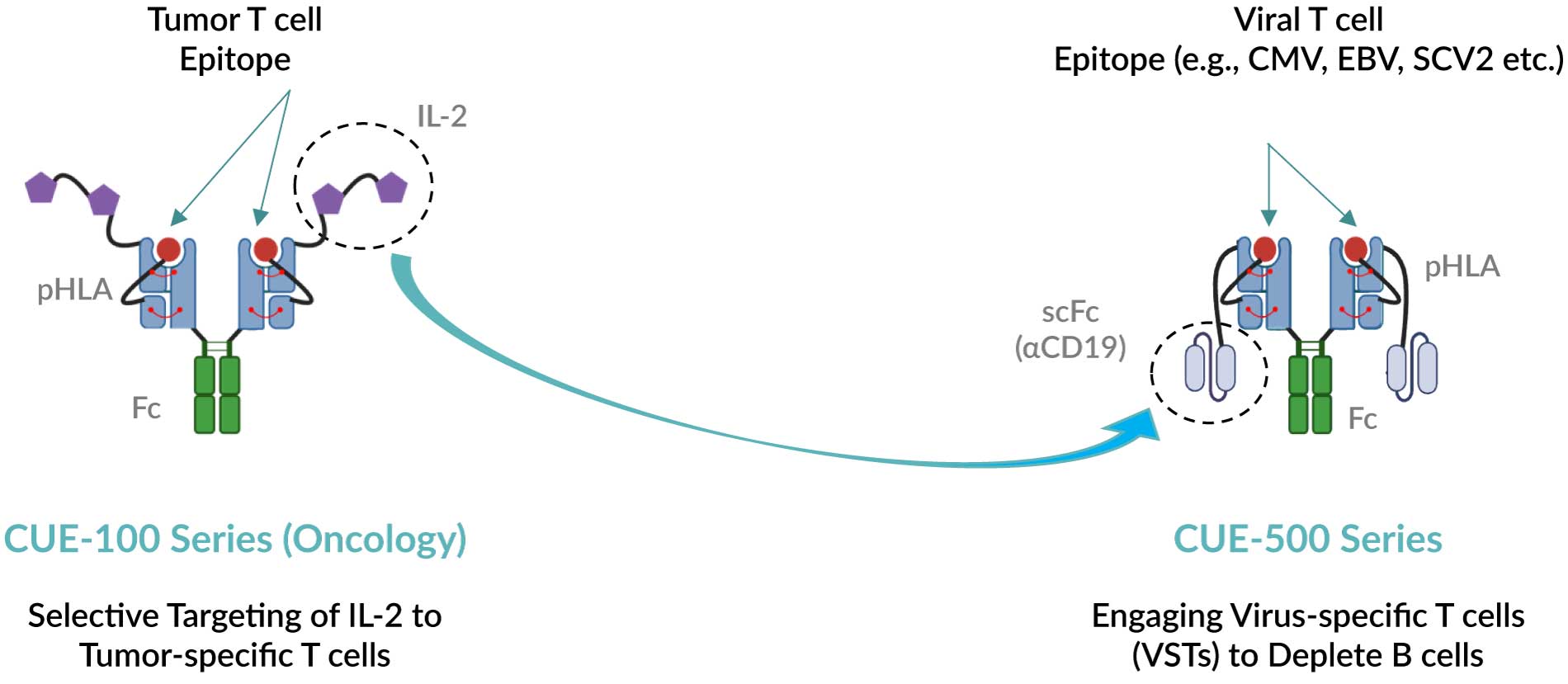
Leveraging a derisked, validated Immuno-STAT framework for T cell-mediated B cell depletion
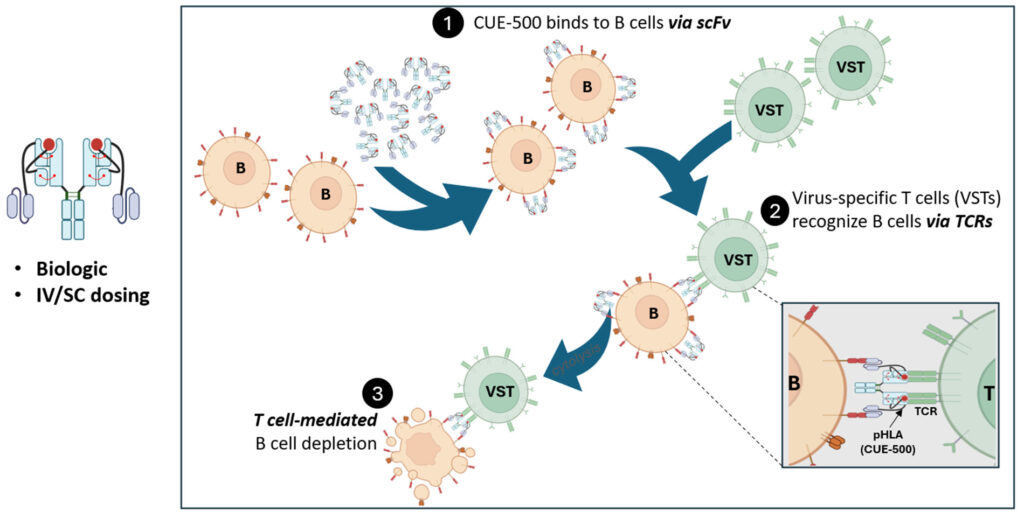
Mechanism of Action
Cue Biopharma welcomes your interest in partnering opportunities for our autoimmune disease programs.
For inquiries, please contact us at BD@cuebio.com.
Current Industry Collaborations in Autoimmune Disease

Cue Biopharma entered into a strategic collaboration and option agreement with Ono Pharmaceutical Co., Ltd. (“Ono”) in February 2023 to support development of CUE-401. Under the terms of the agreement, Ono will financially support CUE-401 research and development activities through a specified option period. After this period, Cue Biopharma will be eligible for development and commercial milestone payments, as well as tiered royalties on sales. Upon this exercise, Ono will receive worldwide rights to develop and commercialize CUE-401 with Cue Biopharma retaining a 50% co-development and co-commercialization right in the United States.

