Harnessing Nature’s “Cues” to Attack Cancer
A crucial breakthrough in the fight against cancer has been the emergence of immunotherapies designed to engage a patient’s immune system.
However, current cancer immunotherapies have several critical limitations:
- They can activate T cells indiscriminately, lowering effectiveness and compromising patient safety and tolerability.
- In most cases, they do not sufficiently increase the right population of T cells necessary to combat the cancer.
- Almost all tumor-specific immunotherapies in development require ex vivo manipulation, i.e., manipulation of T cells outside the body and later infused into the patient, thus limiting clinical applicability.
Our Approach
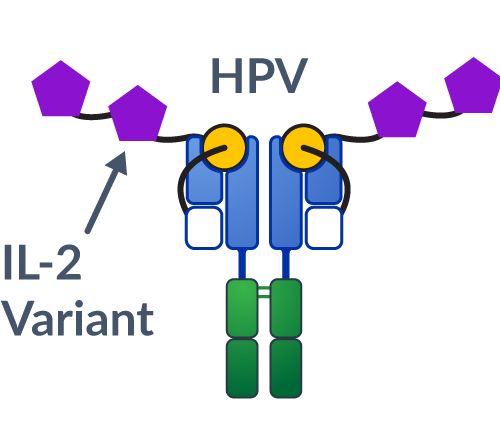
We reverse-engineered how T cells become activated during an anti-cancer immune response, by mimicking the presentation of the natural “cues” or immune signals.
There are on average 10-20 million unique T cell receptors, or unique immune system “addresses,” in our body that can specifically respond to a particular tumor (tumor-specific T cells). Our therapeutics aim to increase the right population of tumor-specific T cells to fight disease.
The key benefit is the ability to provide targeted therapies for cancer that are potentially more effective while avoiding the negative side effects of systemic approaches, that result from attack of healthy tissue.
Learn how our biologics work.
Benefits of Our Approach
- Improved Tolerability and Enhanced Efficacy – Potential to improve overall treatment experience and clinical potential through reduced side effects and improved efficacy
- Conveniently Administered – Standard intravenous (IV) injection delivery with an intermittent dosing schedule that can be administered by specialists and community-based physicians
- Broad Disease Coverage – Access to a growing set of tumor-associated targets and diverse patient populations
- Manufacturability – Uses established development and production processes to support a cost-of-goods and supply chain similar to that of monoclonal antibodies.
Cue Biopharma’s approach represents a breakthrough in the ability to selectively and safely modulate the immune system in a highly controlled and targeted manner directly in the patient’s body, creating potentially life-changing medicines.
Our Platform Technologies
Click on a Platform Technology to learn more.